Drugs Manufacturing and Packaging
Discover safer and more productive technical solutions for your drug production and preservation.
API pharmaceutical
Air Liquide offers gas solutions for the manufacture of API (Active Pharmaceutical Ingredients). The gases are produced and delivered according to GMP (Good Manufacturing Practice), GDP (Good Distribution Practice) and the specifications in the European Pharmacopoeia. Eliminate the uncertainty on the results related to the atmospheres, protect against unwanted reactions with Oxygen and moisture and guarantee that your installations meet the standards of your industrial sector, biopharmaceutical, food.
Biomanufacturing - Cell culture
Upstream bioprocessing applications require a mixture of Oxygen (O₂), Nitrogen (N₂), and Carbon Dioxide (CO₂). This mixture is sparged through the cell culture media to provide replenishment of Oxygen during cell growth. In addition, Carbon Dioxide is used for pH control, while Nitrogen is used to strip excess Carbon Dioxide out of solution and balance the overall flow rate. With Air Liquide, you can be confident in the reliability and quality of the gas supply for your controlled atmospheres.
Cryogenic pelletising
Many scientific studies highlight the role of the gut microbiome in human health and the potential benefits of probiotics. Efficient pelletising of live microbial strains, such as probiotics, often requires very low temperatures. Air Liquide provides cryogenic solutions and tailored equipment to meet your needs.
Inerting, protective atmosphere and purification
Raw materials, API and excipients can be sensitive to Oxygen and moisture in the air. Therefore, they must often be protected with an inert gas to avoid unwelcome reactions, so that the active properties of the drug can be preserved. Inertisation, protection and purification with Nitrogen also contribute to reducing the risk of ignition and explosion when, for example, storing flammable solvents and milling powder. Air Liquide's solutions, which include gas, equipment and services, give you optimised Nitrogen consumption and precise control.
Reduction of particle size
Reducing the particle size of the API and excipients plays an important role in the properties and quality of the final product, i.e. solubility, uniform content and stability. Air Liquide offers a cryo-milling application where the material is cooled to low or very low temperatures with a cryogenic liquid, usually liquid Nitrogen, so that the particles have a dense size distribution.
Process temperature control
Certain steps in the manufacture of API, such as lyophilization and freeze drying, require low or very low temperatures. Liquid Nitrogen can be used to cool the process, either directly or indirectly as it is vaporised and heated.
Freeze drying is often the process of choice for stabilizing therapeutic proteins or vaccines. Cryogenic freezing is an environmentally friendly alternative to conventional refrigerants (CFCs) in freeze drying, as the production cycle can be reduced.
Pharmaceutical formulation, packaging and distribution
Air Liquide offers solutions that can help you protect sensitive products from exposure to Oxygen, optimise the composition of the drug, and maintain a cold chain for your clinical and biological samples. We design the gas installation by collaborating with you and ensure that your gas supply best suits your requirements and needs.
Logistics for the cold chain
Temperature variations can affect the stability of a medicine and thus its healing effect. Temperature-sensitive pharmaceutical and diagnostic products must be kept stable during distribution and arrive at their final destination without damage.
Air Liquide offers solutions that include cold chain logistics with dry ice or liquid Nitrogen. Even very sensitive deliveries of e.g. clinical samples, vaccines, diagnostic and biological equipment can be stored and transported without damage.
Packaging
Some medicines are sensitive to oxygen and moisture in the air. Therefore, they often have to be protected with an inert gas in order for them to maintain their healing effect. Some therapeutic proteins are packaged in a Nitrogen atmosphere during fill-finish operations to extend shelf life.
As the gas comes into direct contact with the final pharmaceutical product, it is important that the gas supply is reliable and complies with current regulations.
Waste water treatment
Our recommended gas and equipment solutions for your laboratory
Our experts will help you choose the right gas for your analysis and give you advice on the use of related equipment. In this way, it is possible to obtain reliable and high-quality measurement results in complete safety.
Related Equipment
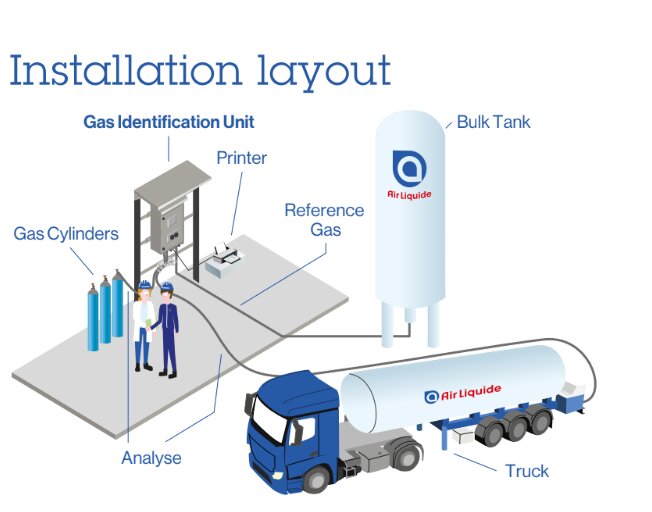
Gas Identification Unit (GIU)
An innovative device to quickly measure process incoming gases (e.g. N₂, O₂, CO₂) used in the pharma and bioscience industries . It is user-friendly, safe, and does not require calibration for your GMP compliant operation.
Why Choose Us
-
Industry Leader
We are a global leader in gases, technologies and services for Industry and Health. -
Reliability
Our solutions are reliable, reproducible and in compliance with stringent and ever-evolving regulations. -
Expertise
Leverage our global network of industry experts tailored to your unique challenges. -
One-Stop Solution Provider
Access a comprehensive suite of products, supply modes, and services designed for your specific requirements. -
Extensive Infrastructure Network
Benefit from our robust production and filling infrastructure, including extensive pipeline networks. -
Focus on Quality
Expect nothing less than the highest standards in process and product quality. -
Sustainability
Embrace our solutions that support your highest performance while minimising environmental impact.
Contact Us
Let our team get back to you on your specific enquiry.